The CoolStat – completely new way to manage patient temperature.
Designed to provide effective cooling that is simple, safe, and easy-to-use.
COOLSTAT
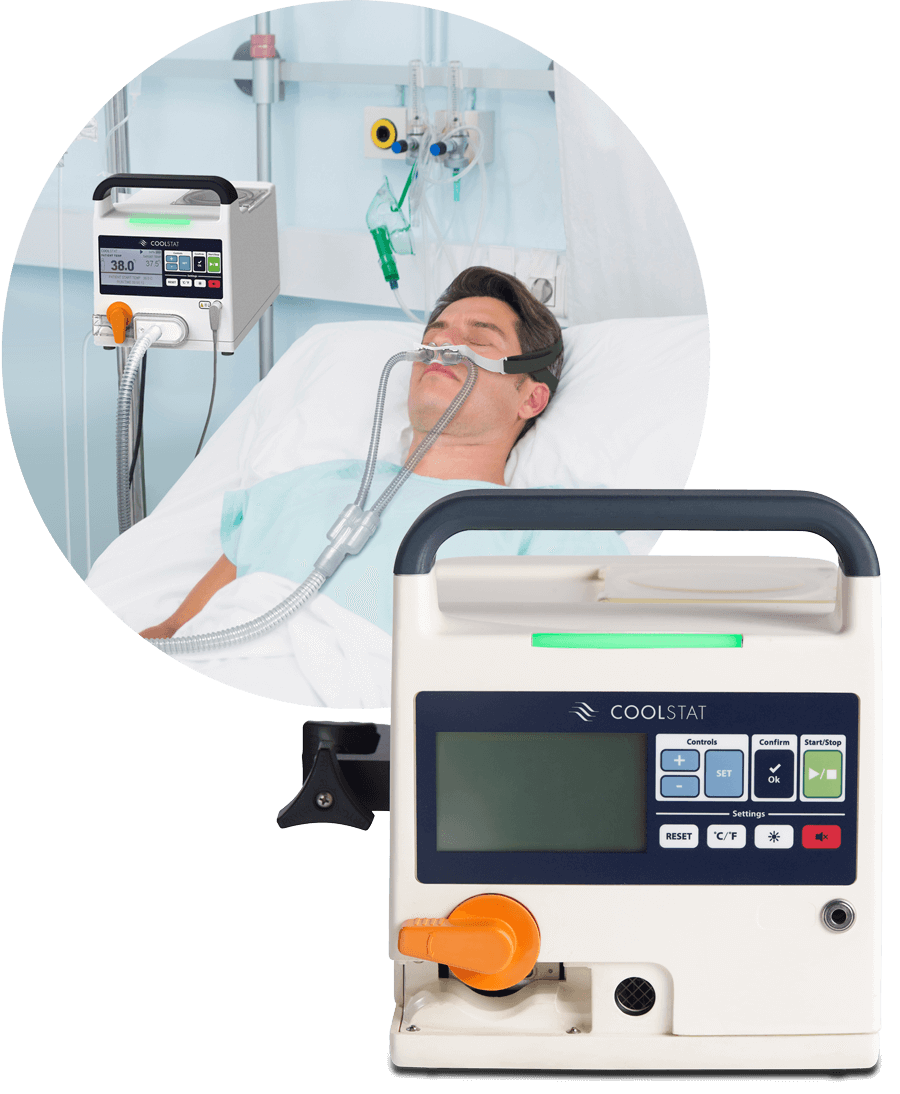
The CoolStat is a completely new way to manage patient temperature. Unlike other cooling systems, the CoolStat does not use cumbersome cooling pads or large refrigeration carts. The CoolStat is the ultimate in simplicity. It cools using only dry, room temperature air, based on a simple technique referred to as evaporative cooling.
SCIENCE APPLIED TO COOLING
HOW IT WORKS
The CoolStat blows dry, room temperature air into the nose and over the nasal turbinates in a unidirectional fashion before flowing freely out of the mouth. The nasal turbinates are highly vascularized, mucus-containing membranes with a large, convoluted surface area. As the dry air from CoolStat flows over the moist turbinates, it induces the liquid water and mucus on the turbinates to change phase, from a liquid to a gas. This phase change pulls energy out of the body, causing systemic cooling.

COOLSTAT FEATURES
Easy to install and operate
Only portable device - cooling anywhere!
Potential for expanded markets and IFUs
Potential for use prophylactically in awake patients
Designed to be comfortable for patient
Designed to reduce or eliminate shivering response
Designed to provide clinically effective cooling,
Very little infection risk (non-invasive)
Lower cost than other targeted temperature management (TTM) systems
FAQ SECTION
How is the CoolStat Used?
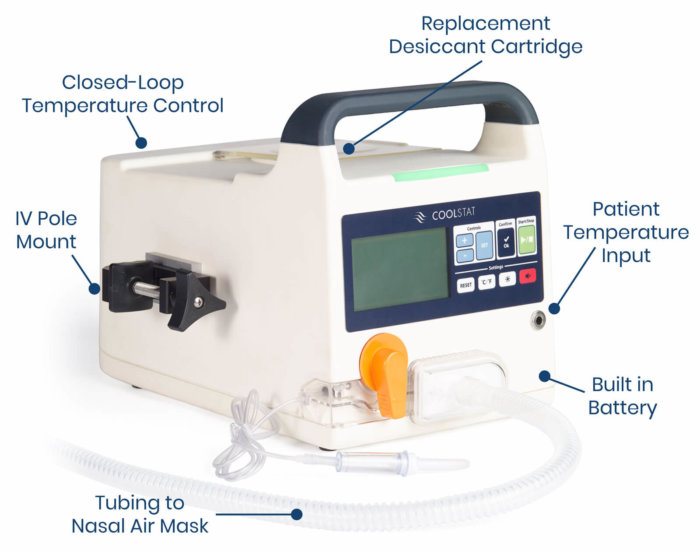
The CoolStat device is small, lightweight and portable, able to move throughout the hospital with the patient. The device generates a source of filtered air that is delivered to the patient via a single-use air tubing set. The only interface with the patient is a simple nasal mask. The CoolStat includes a closed-loop temperature control system. That means that the operator of the device can select a target temperature, and the CoolStat is designed to automatically cool and hold the patient at the target temperature.
What indications is the CoolStat designed to treat?
The CoolStat is not currently approved by the FDA as a therapy for any indication. The device is currently being used in clinical studies in neurogenic fever (central fever) that can develop following a stroke, traumatic brain injury, seizures and metabolic encephalopathy. We expect the clinical studies to be completed by the end of 2020. We are also exploring the use of the CoolStat to initiate mild hypothermia in a pre-hospital setting, post sudden cardiac arrest, and we have seen positive early results using the CoolStat to reduce or eliminate the pain from migraine.
PUBLICATIONS
Targeted Temperature Management: Degrees of Separation
Rapid Induction of Therapeutic Hypothermia Using Transnasal High Flow Dry Air
Efficacy and Safety of Transnasal CoolStat Cooling Device to Induce and Maintain Hypothermia